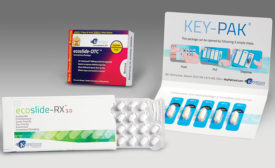
Blister Packaging
Materials Technology: Blisters & Cards
Factors to consider for packaging over-the-counter and prescription medications include safety, adherence and product quality.
Read More
Cover Story
A Prescription for Pharmaceutical Packaging
Serialization, safety and security head a long list of challenges for the pharmaceutical packaging industry in 2018
April 1, 2018
HCPC Column
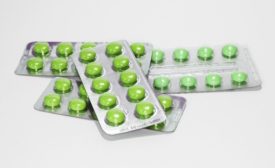
FDA Favors Blister Packs In Battle Against Opioids
Benefits of unit-dose blister packaging include safeguarding medication efficacy, improved supply chain security and aiding medication adherence.
March 15, 2018
Healthcare Compliance Packaging Council Column
The Role Packaging Plays in the Opioid Crisis
January 16, 2018
Materials Technology: Protective packaging
A global role for protective packaging
Ecommerce is growing rapidly and with more consumers buying online, protective packaging is crucial.
December 19, 2017