Pharma/Medical Packaging
Materials Technology
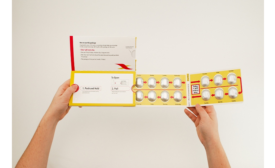
Blister packaging plays dual role as protector and barrier
Protection starts on the outside
May 12, 2017
Keep the info flowing with our eNewsletters!
Get the latest industry updates tailored your way.
JOIN TODAY!Copyright ©2025. All Rights Reserved BNP Media.
Design, CMS, Hosting & Web Development :: ePublishing