Healthcare Compliance Packaging Council Announces Compliance Packages of the Year








As part of its mission to improve patient adherence and outcomes through packaging, the Healthcare Compliance Packaging Council (HCPC) is excited to announce the winners of its annual Compliance Package of the Year competition. The winners were announced at the organization’s RxAdherence2017, Strategies to Improve Outcomes conference held May 2 in Florham Park, NJ. HCPC’s Compliance Package of the Year competition has been an annual event since 1995 and recognizes the most innovative pharmaceutical packages designed to improve patient adherence for the preceding market year. In addition to recognizing innovative compliance packages commercially available, the HCPC encourages innovation and seeks to highlight inventive packaging designs to promote patient adherence with its Innovative Design category. This year’s winners are:
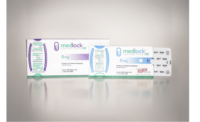
HCPC 2016 Compliance Package of the Year: Merck ZepatierTM Medication Package from WestRock
The Zepatier® Medication Package promotes patient medication adherence using a custom design that integrates a calendared blister clearly labeled by day on each individual pill cavity, highlighting the two week dosing regimen (2 two-week packages make up a monthly supply of Zepatier). Visual cues prompt medication taking and tracking. A 5/6th panel provides space for medication information, dosing instructions, and how to navigate the calendar. In addition, branding for Merck / Zepatier is easily seen on the outer package’s readable, flat space.
“WestRock and Merck partnered to design the Zepatier package to help HCV patients adhere to their medication,” said John Grinnell, VP, sales, health, beauty, & adherence, WestRock. Packaging is the one tool that stays with the patient throughout the medication journey, making it uniquely effective in providing regimen support.”
The Zepatier package builds on WestRock’s legacy of designing medication packaging that’s proven to enhance adherence. WestRock’s enhanced adherence packaging is shown to achieve an 80% PDC with up to 37% more patients versus standard vials.
When reviewing, one judge advised the package had a “Very clearly explained regimen with an easy-to-read font type and size. The red color is used appropriately to emphasize important instructions. With clearly stated details printed on the inside of the fold-open package and arrows guiding the patient through each daily dose, the package encourages compliance by keeping the regimen as simple as possible.”
HCPC 2016 Compliance Package of the Year First Runner Up: Braeburn Pharmamceuticals’ Probuphine Implant Kit, Manufactured by Sharp Packaging Solutions
The Sharp Global Design team was asked to design and construct a packaging solution for Probuphine® (buprenorphine) implant] in 2014 that would contain multiple components of various sizes, in a physician-friendly package. The challenge was to ensure that the design and usability of the packaged kit would effectively contribute to the correct use and administration of the drug implant by physicians. The Sharp Design team partnered with Braeburn Pharmaceuticals during many stages of the product packaging development, including design, prototype development and finally, commercial production.
Comments provided by the judges included “This implant kit seems very comprehensive for physicians, as well as for patients. The kit demonstrates the critical role that packaging and labeling can play in guiding professionals through safe and effective product use. The font and colors printed on the package labeling are clear and promote comprehension, and the patient materials are also very clearly stated. The 3-D package design successfully accommodates a significant amount of product components and labeling materials in reasonable footprint. There are sufficient warnings and tracking features to further guide both professionals and patients for appropriate product use and security.
The Probuphine is now in commercial production in Sharp’s facility in Allentown, PA.
HCPC 2016 Compliance Package of the Year First Runner Up: CVS Store Brand OTC Compliance Pack
Many Over-The-Counter (OTC) products are used to treat the side effects of prescription medications – pain, constipation, etc. CVS was looking for a new way to guide patients to these OTC treatments in a way which also introduced them to the value of CVS Store Brand products.
They selected WestRock’s Dosepak Express package, in conjunction with a WestRock merchandising display located under the pharmacy checkout counter, to accomplish this objective. The private labeled, store brand products were launched in the stores in September, 2016, with 10 items currently in the program. Each package contains 14 tablets.
While the term “compliance” is typically not associated with “take-as-needed” OTC products, the Dosepak Express format provides the convenience, ease of use, and portability necessary to promote proper adherence use by patients, while offering ample billboard space to accomplish CVS’ marketing goals.
Some specific features offered:
- Child-Resistance features in the outer pack allows elimination of difficult to use peel-push OTC blisters
- Robust pack construction promotes portability and product protection
- Connection of blistered drug product to the outer package throughout use maintains CVS’ brand identity throughout the life of the product
One judge commented, “It is good to see patient-friendly packaging used for store-brand products, which can increase product value for the patient. The expanded content label includes helpful information in a very readable font size. The tamper evidence is helpful and clear for the patient. I’m not sure this is a true compliance package as there doesn’t seem to be much regimen guidance, but that could be because the product is to be taken as needed.”
2016 HCPC Innovative Design Award: Med-ic® Syringe Pack equipped with Med-ic® ECM® (Electronic Compliance Monitor) Technology, Submitted by Intelligent Devices SEZC Inc (IDI) for Information Mediary Corporation
Med-ic® is an electronic compliance monitor that addresses non-compliance with prescribed medication by monitoring medication taking behavior in real time, tracking each dose removed by the patient. The entry submitted was a syringe package that not only would record the date and time a syringe was removed, but it allowed the patient or caregiver to indicate the injection location on a body part, upper arm, abdomen, buttocks or thigh. Med-ic can also monitor storage temperature to ensure product integrity. Med-ic creates a smart medicine package which keeps a history of the exact time the patient removed each tablet, capsule or syringe and can fit child-resistant packaging.
One judge was pleased with the technology with some caveats. “This is a very ambitious packaging system attempting to promote patient compliance as well as ensure product safety and security. The downloadable app and interactive package should be very helpful for patients. The embedded electronic tag will be very effective in registering package opening, and I wonder whether it could also play a role in tamper evidence. The status indicator light for demonstrating product temperature safety will also be very helpful to patients. The numbering system for indicating injection site should be helpful, too, but without adjacent labeling support, it could be slightly confusing. Patients might be tempted to push the buttons before fully understanding their purpose. There is a lot of potential for on-package labeling instructions, product branding, and other compliance-prompting features.”
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!