For the convenience and ease of use blister packaging provides consumers, the creation of such packaging isn’t as simple. From developing appropriate machinery to sourcing the right materials, blister packaging brings a variety of challenges that suppliers are constantly trying to mitigate.
As an example, Pharmaworks has developed blister machinery that can handle 100% recyclable mono-materials, marking a significant improvement when it comes to the sustainability of these operations.
Additionally, PulPac, PA Consulting and Optima collaborated to develop bespoke machinery that can create blister packaging from Dry Molded Fiber. The collaboration allows this practice to operate at a large scale while also contributing to a circular economy.
Finally, Aptar CSP Technologies announced that its N-Sorb nitrosamine mitigation solution was accepted into the U.S. Food & Drug Administration’s Emerging Technology Program, which promotes the adoption of innovative approaches to pharmaceutical product design and manufacturing.
Learn more about these innovations below.
Pharmaworks Develops Blister Machine for 100% Recyclable Mono-Materials

Pharmaworks can also upgrade existing blister machines to make them compatible with mono-materials. Courtesy of Pharmaworks
Pharmaworks, a ProMach product brand, recently debuted its new capability to handle recyclable, mono-material packaging films on its blister machines at Interphex. In addition to offering their entire fleet of available blister packaging systems ready to run these challenging films, the company is offering upgrades for installed Pharmaworks blister machines in the field and can rebuild blister machines from other suppliers to handle these new sustainable films.
PulPac, PA Consulting and Optima Collaborate on Machinery for Dry Molded Fiber
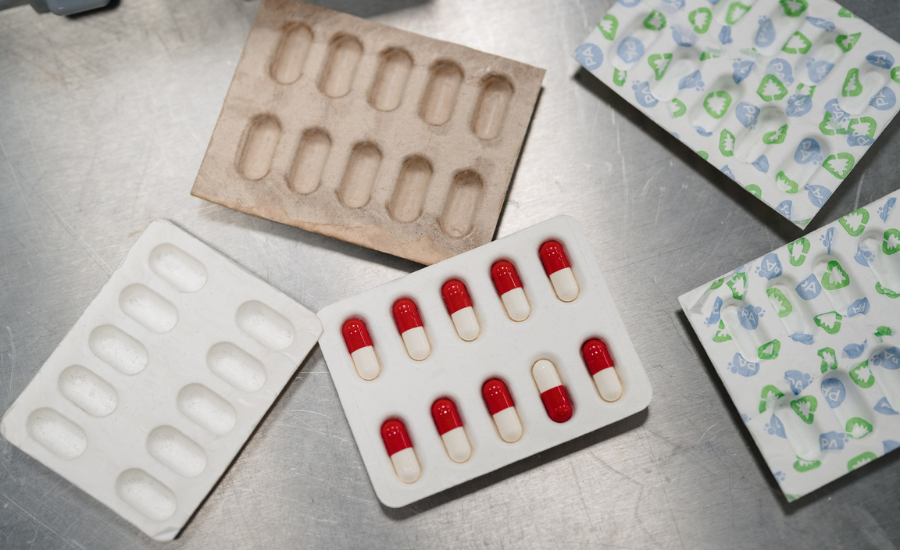
The collaboration allows for the use of sustainable materials while maintaining high-level production. Courtesy of PulPac
PulPac, PA Consulting, and Optima have partnered to develop bespoke machinery for advanced Dry Molded Fiber applications.
The partnership aims to facilitate the industrialization of complex products such as coffee capsules and blister packaging. By integrating PulPac’s pioneering Dry Molded Fiber technology with PA Consulting’s product development expertise and Optima’s renowned bespoke machine building capabilities, the collaboration will drive the scalability and high-throughput production of commercially viable, advanced Dry Molded Fiber applications, supporting PulPac’s and PA Consulting’s Blister Pack Collective and other initiatives for advanced Dry Molded Fiber packaging.
Aptar Earns Acceptance to FDA's Emerging Technology Program for N-Sorb Solution

N-Sorb can eliminate the need for pharmaceutical companies to reformulate their products. Courtesy of Aptar CSP Technologies
Aptar CSP Technologies, part of AptarGroup, Inc. and a leader in active material science, announced that its N-Sorb nitrosamine mitigation solution has been accepted into the U.S. Food & Drug Administration’s (FDA) Emerging Technology Program (ETP), which helps promote the adoption of innovative approaches to pharmaceutical product design and manufacturing.
N-Sorb leverages Aptar CSP Technologies’ proven 3-Phase Activ-Polymer™ platform technology to address the pressing issue of N-nitrosamine impurities in pharmaceuticals. These impurities, classified as probable human carcinogens, have raised significant regulatory concerns and prompted numerous drug product recalls. Nitrosamines can form during drug product storage or transport, posing risks to patient health.





